Other than this, the rising want for cleaner burning fuels and feedstocks for chemical production is contributing to the growth of the market within the area. In line with this, favorable government initiatives to maintain sustainability are propelling the growth of the market. In addition to this, the wide availability of agricultural and biomass assets is bolstering the expansion of the market in the Asia Pacific area. Numerous companies on this trade are specializing in improving manufacturing processes, exploring new feedstock sources, reminiscent of biomass, and creating superior DME applications, particularly within the automotive and energy sectors. According to this, major manufacturers are diversifying their product portfolios by developing varied grades and formulations of DME, equivalent to high-purity DME for chemical processes and DME blended with liquefied petroleum gasoline (LPG) for cleaner fuel applications. Other than this, they're focusing on their dedication to maintaining sustainability by promoting DME as an eco-pleasant fuel and feedstock possibility. They are also actively advertising renewable DME merchandise that possess reduced carbon footprint and decrease emissions to attract environmentally aware customers.
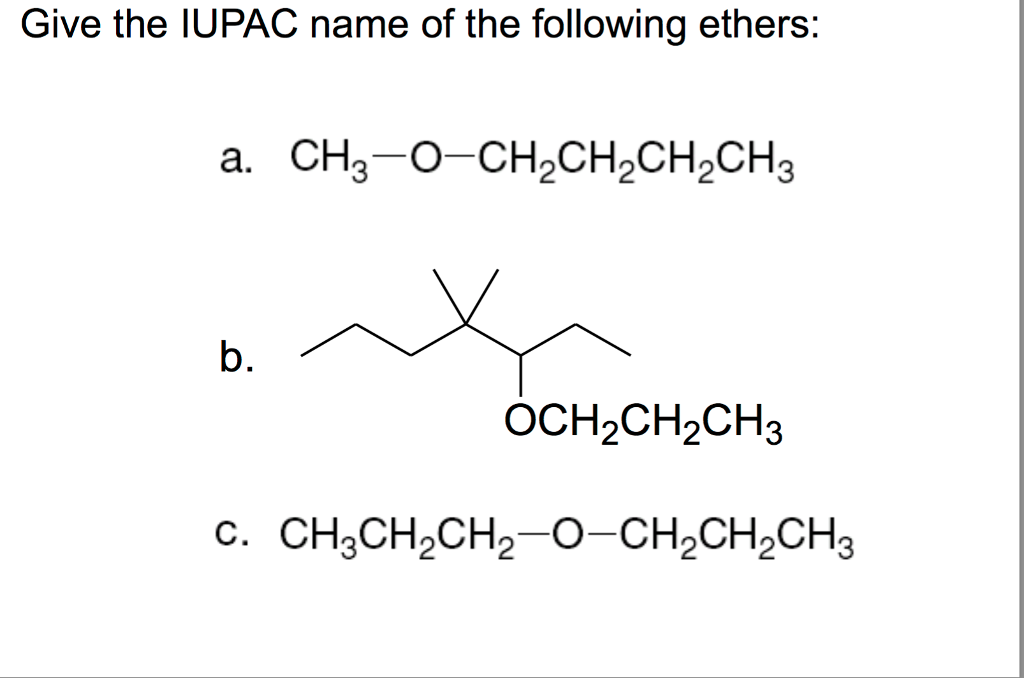
It is dedicated to sustainability and has carried out varied measures to scale back its environmental influence. The corporate has also applied a waste management program to attenuate the amount of waste generated by its operations. Additionally it is involved in various social initiatives, comparable to neighborhood development initiatives and disaster relief efforts. Describe the structural distinction between an alcohol and an ether that affects bodily characteristics and reactivity of every. 2. Name easy ethers. 3. Describe the construction and uses of some ethers. With the general method ROR′, an could also be thought of a derivative of water through which each hydrogen atoms are changed by alkyl or aryl teams. Easy ethers have easy widespread names, formed from the names of the teams connected to oxygen atom, adopted by the generic title ether. For example, CH3-O-CH2CH2CH3 is methyl propyl ether. If both teams are the identical, the group identify should be preceded by the prefix di-, as in Dimethyl ether price ether (CH3-O-CH3) and diethyl ether CH3CH2-O-CH2CH3. The IUPAC system names ethers by naming the longer end as an alkane and treating the shorter end as as an "alkoxy" substituent.
If we glance into the periodic desk, we will test that Carbon has a valency of 4, Oxygen has a valency of 6, and Hydrogen has only 1 valence electron. A molecule of dimethyl ether has 20 valence electrons. We'd like to search out out the central atom of the given molecule. In natural chemistry, one of an important key points to remember while sketching the electron dot structure is that hydrogen atoms will usually take the terminal positions irrespective of the comparison within the electronegativity values. We will see that oxygen has acted because the central atom on this molecule flanked by two methyl groups at both sides. Tapp, J.S.; Steacie, E.W.R.; Maass, O., Density of a Vapor in Equilibrium with a Liquid Near the Important Temperature., Can. Cardoso, E.; Coppola, A.A., Experimental researches on some thermal properties of gas I the densities of coexisting phases of methyl ether, J. Chim. Ambrose, D.; Ellender, J.H.; Sprake, C.H.S.; Townsend, R., Thermodynamic properties of organic oxygen compounds XLIII. What's the IUPAC title for every ether? Simple ethers even have frequent names, formed from the names of the teams hooked up to oxygen atom, followed by the generic name ether. If both groups are the same, the group title should be preceded by the prefix di-. What is the widespread title for each ether?
At present, the rising adoption of DME as a feedstock within the production of different chemicals, similar to dimethyl sulfate and acetic acid, is contributing to the growth of the dimethyl ether (DME) market. According to this, the growing employment of DME, as it's a cleaner different to traditional fossil fuels, is strengthening the growth of the market. Furthermore, the rising considerations about air high quality and greenhouse gasoline (GHG) emissions among the many lots all over the world are bolstering the growth of the market. In addition, the rising demand for power and chemical products because of fast industrialization throughout the globe is offering profitable growth alternatives to trade investors. Moreover, governing agencies of a number of countries are implementing stringent rules and regulations on harmful emissions, which is supporting the expansion of the market. The rising desire for cleaner power sources and fuels throughout various industries across the globe is contributing to the expansion of the dimethyl ether market dimension. According to this, persons are increasingly specializing in sustaining environmental sustainability. DME is a viable alternative resulting from its decrease carbon footprint as in comparison with traditional fossil fuels.
DME has, up to now, been considered for use as a human anesthetic. It needs to be famous that this chemical can produce cardiac sensitization just like the consequences of epinephrine. Dimethyl ether is an aggressive solvent and should affect the gasket supplies used in aerosol packaging. Oxidizing agents, acetic acid, natural acids, and anhydrides shouldn't be used with dimethyl ether. This consists of oil and gas, automotive, power era, cosmetics, and others. According to the report, automotive represented the biggest segment. DME is quickly gaining reputation in the automotive sector as a substitute gasoline source. It has clean-burning traits, excessive cetane quantity, and low emissions profile, which makes it an attractive option for decreasing environmental impact in transportation. In accordance with Procurement Resource, the prices of dimethyl ether (DME) will continue to indicate a fluctuating trend depending upon the worth of its feedstock and downstream demand. In the course of the early levels of the H2 of 2023, there was a notable enhance in the price of uncooked supplies like sulphuric acid and varied different chemicals within the Indian market. April 2024: CSIR-IICT and BHEL signed a Memorandum of Understanding (MoU) for creating a method that converts carbon dioxide (CO2) into DME. This mission is a component of a broader Carbon Capture and Utilization (CCU) initiative by the Department of Science & Technology, Authorities of India. Utilizing a direct catalytic conversion methodology, the purpose is to absorb CO2 and transform it into DME. The research report research provides the newest info on the market drivers, challenges, and alternatives in the worldwide dimethyl ether market.











